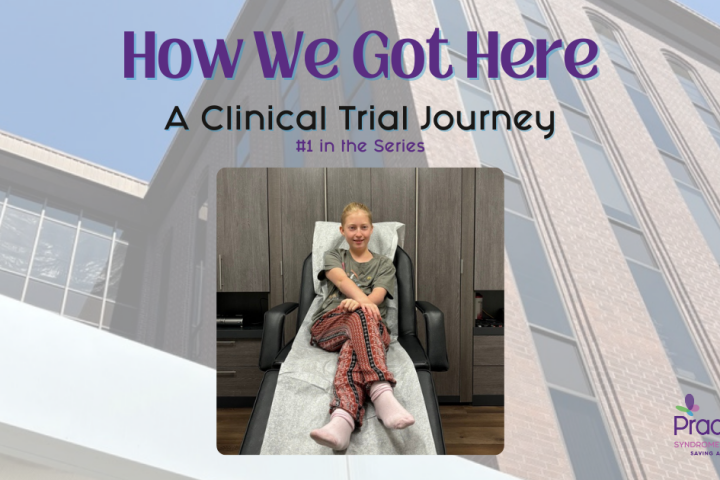
contributed by Anne Fricke I lay awake in a pre-dawn haze the morning Freya was to take her first pill. We had been to Southern California twice already, a combination of 7 flights up and down the coastline, numerous hours of travel, and far too many airport meals, and I was still momentarily on the...
Category: Research
Update on Phase 3 COMPASS PWS Study from Acadia
September 24, 2024 Dear Prader-Willi Syndrome Community, We are pleased to share an update on the 12-week, pivotal Phase 3 COMPASS PWS study evaluating the efficacy and safety of carbetocin nasal spray (ACP-101), an investigational drug, for the treatment of hyperphagia in Prader-Willi syndrome. The study was initiated in the United States in November 2023...
FDA Advisory Committee to Review DCCR as Treatment for Hyperphagia in PWS
The FDA has announced its plan to conduct an Advisory Committee Meeting as part of its review of Soleno Therapeutics’ DCCR New Drug Application. The FDA convenes Advisory Committees to provide independent expert advice that contributes to the agency’s regulatory decision-making. As part of the Advisory Committee Meeting, interested community members are encouraged to share...
FDA Accepts Application for New Drug DCCR, Moves to Priority Review
Exciting news for the Prader-Willi syndrome (PWS) community! Soleno Therapeutics has announced that the FDA has accepted their new drug application (NDA) for DCCR, a drug designed to treat hyperphagia in individuals with PWS aged 4 and older. This acceptance is a major first step, and the FDA has granted Priority Review, recognizing the potential...
Survey Results on the Aging Adult with PWS
Contributed by Barb Dorn, RN, BSN As I began my research looking at specific health issues in the aging adult with PWS, I soon learned that there was not much information on this topic. I did find a few articles that documented clinical evidence for early signs of aging. As far as dementia, I found...
Aging Research in Prader-Willi Syndrome
Compiled by Barb Dorn, RN, BSN People with PWS are growing old. Many of this may be the result of our increased knowledge in supporting and caring for the person with PWS. We have learned to replenish hormone deficiencies and manage their diet and food security. We have identified critical health issues and know that...
Soleno Therapeutics Submits New Drug Application to FDA for PWS Treatment
On June 28, 2024, Soleno Therapeutics announced the company officially submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for DCCR (diazoxide choline) extended-release tablets. This new treatment targets Prader-Willi syndrome (PWS) in individuals aged 4 and older with hyperphagia. CEO Anish Bhatnagar, M.D., says, “Submission of the DCCR NDA to...
Help Needed: Caregivers of Children with Prader-Willi Syndrome and Repetitive Verbal Behavior
Kasey Bedard, PhD, and her team at The Chicago School are seeking caregivers of children diagnosed with Prader-Willi Syndrome (PWS) who exhibit repetitive verbal behavior for a research study at The Chicago School. This study, part of a dissertation project, aims to test interventions that caregivers can implement at home. Study Details: – Duration: 2-3...
Fueling Hope: Aardvark Therapeutics’ $85M Boost Powers Breakthrough PWS Treatment
Aardvark Therapeutics has just hit a major milestone with an exciting $85 million Series C financing round! Led by Decheng Capital, with additional participation from several investors, including PWSA | USA, this funding is set to drive the development of ARD-101, a groundbreaking treatment for hyperphagia in Prader-Willi syndrome patients. ARD-101 showed promising early trial...
Request for Prader-Willi Syndrome Research and Mini-Fellowship Grant Applications
PWSA | USA is currently seeking research project applications with direct impacts on individuals and families affected by PWS. We are offering mini-fellowship grants to support providers in enhancing their understanding of PWS through clinical proctorships. Research priorities include expanding knowledge about PWS, applying therapies, and attracting new providers and investigators to the field. Funding...
Breaking Ground: FDA Grants Breakthrough Designation for PWS Drug Development
Big news! Soleno Therapeutics has announced a groundbreaking achievement: diazoxide choline (DCCR) has been granted Breakthrough Therapy Designation by the FDA for Prader-Willi syndrome (PWS). This marks a significant milestone as the FIRST-EVER designation for a drug developed for PWS. The designation underscores the FDA’s recognition of PWS as a serious condition and the potential...
Harmony Biosciences Initiates TEMPO PWS Study
Harmony Biosciences Holdings, Inc. is seeking participants for its TEMPO study, a global Phase 3 trial investigating pitolisant as a potential treatment for excessive daytime sleepiness (EDS) in individuals aged six years and older with Prader-Willi syndrome (PWS). Pitolisant is a medication that could help manage sleepiness and behavioral issues in people with PWS. There...
New PWS Clinical Study: Free Informational Webinar with Harmony Biosciences
Join PWSA | USA and the team from Harmony Biosciences on Tuesday, March 12th at 8:00 p.m. EST / 5:00 p.m. PST to learn more about the upcoming Phase 3 registrational TEMPO study, a randomized, double-blind, placebo-controlled, multicenter, global clinical study that will further assess the safety and efficacy of pitolisant in patients with PWS,...
Pitolisant Receives Orphan Drug Designation
Pitolisant from Harmony Biosciences Receives Orphan Drug Status from FDA for Treatment of PWS The FDA has granted Orphan Drug status to pitolisant, the trial drug from Harmony Biosciences to treat excessive daytime sleepiness (EDA) and behavioral disturbances. The designation shows that the FDA considers pitolisant to be a promising treatment for people with PWS. ...
Neuren Pharmaceuticals Opens Third Trial Site for Phase II PWS Study
Neuren Pharmaceuticals is pleased to announce their third site participating in their Phase II, Open Label, PWS Study (Neu-2591-PWS-001) is now open for screening! Important information regarding this exciting milestone: Three sites are now open to enrollment! Rare Disease Research (RDR), located in Atlanta, GA, and Uncommon Cures, located in Chevy Chase, MD (8 miles outside of Washington, D.C.) and...
Neuren Pharmaceuticals Opens Second Trial Site for Phase II PWS Study
Neuren Pharmaceuticals is pleased to announce their second site participating in their Phase II, Open Label, PWS Study (Neu-2591-PWS-001) is now open for screening! Important information regarding this exciting milestone: Two sites are now open to enrollment! Rare Disease Research (RDR), located in Atlanta, GA, and Uncommon Cures, located in Chevy Chase, MD (8 miles outside...
Acadia Pharmaceuticals has Announced the Initiation of its Phase 3 Study for Nasal Carbetocin to Treat Hyperphagia
This week, Acadia Pharmaceuticals announced the initiation of its Phase 3 COMPASS PWS study! This study focuses on evaluating the efficacy and safety of carbetocin nasal spray (ACP-101) for treating hyperphagia in Prader-Willi syndrome. The COMPASS PWS trial is a 12-week, double-blind, randomized, placebo-controlled global Phase 3 trial, with approximately 170 participants aged five to...
PWSA | USA Announced as Harmony Biosciences’ 2023 Patients at the Heart Grant Recipient
About Harmony Biosciences Patients at the Heart Grant Via Harmony Biosciences Press Release: Harmony Biosciences Holdings, Inc. (“Harmony”) (Nasdaq: HRMY), a pharmaceutical company dedicated to developing and commercializing innovative therapies for patients with rare neurological diseases, has selected the latest round of nonprofit organizations for its Patients at the Heart and Progress at the Heart...
Pitolisant Shows Positive Secondary Outcomes in Phase 2 Study
Exciting news for the Prader-Willi syndrome (PWS) community! Newly unveiled data from Harmony Biosciences' phase 2 study reveals promising impacts of pitolisant (Wakix) on PWS patients with excessive daytime sleepiness (EDS). Harmony Biosciences plans to kick off its phase 3 TEMPO study later this year, aiming to dive deeper into the potential of this treatment...
Acadia Pharmaceuticals Unveils New COMPASS PWS Study Website
This new website gives in-depth details about Acadia Pharmaceuticals COMPASS PWS Study, including a brief video about the study, eligibility for participation in the study, what to expect at study visits, and a research site locator. Click the button below to enter the website. ABOUT THE COMPASS PWS STUDY: The COMPASS PWS study aims to...
Harmony Biosciences Reveals Encouraging Data from Phase 2 Pitolisant Study in PWS Patients
Harmony Biosciences has presented new secondary endpoint data concerning the use of pitolisant, an investigational drug, for the treatment of excessive daytime sleepiness (EDS) in Prader-Willi syndrome. According to a recent press release Harmony Biosciences released, the Phase 2 signal-detection study showed improvements in behavioral disturbances, especially in the higher-dose pitolisant group, as well as...
Soleno Therapeutics Reports Positive Results from DCCR Study C602 for Prader-Willi Syndrome
Soleno Therapeutics, Inc. has revealed positive outcomes from the randomized withdrawal phase of Study C602, an extended treatment study of DCCR (Diazoxide Choline) Extended-Release tablets for Prader-Willi syndrome (PWS). The results, which showed a significant improvement in hyperphagia-related behaviors in the DCCR group compared to the placebo group, support Soleno's plan to submit a New...
Neuren Pharmaceuticals is Happy to Announce the First Site Participating in Their Phase II, Open Label, PWS Study (Neu-2591-PWS-001) is Now Open for Screening!
Important information regarding this exciting milestone: Rare Disease Research (RDR), located in Atlanta, GA, is now welcoming children with PWS and their families to their clinicfor screening into this trial. The duration of active treatment in this study is 13 weeks. In a preclinical study in animals, physiological and behavioral symptoms were normalized within six...
Harmony Biosciences Issues Statement Regarding Confidence in Pitolisant Drug
Harmony Biosciences has reaffirmed its confidence in the strength of WAKIX® (Pitolisant) patents, after receiving a positive ruling from the U.S. Patent and Trademark Office (USPTO) rejecting the request for reexamination. WAKIX® is used to treat excessive daytime sleepiness (EDS) or cataplexy in adults with Narcolepsy. Read Harmony Biosciences' community-facing statement below: We are pleased...




















 Perry A. Zirkel has written more than 1,500 publications on various aspects of school law, with an emphasis on legal issues in special education. He writes a regular column for NAESP’s Principal magazine and NASP’s Communiqué newsletter, and he did so previously for Phi Delta Kappan and Teaching Exceptional Children.
Perry A. Zirkel has written more than 1,500 publications on various aspects of school law, with an emphasis on legal issues in special education. He writes a regular column for NAESP’s Principal magazine and NASP’s Communiqué newsletter, and he did so previously for Phi Delta Kappan and Teaching Exceptional Children. Jennifer Bolander has been serving as a Special Education Specialist for PWSA (USA) since October of 2015. She is a graduate of John Carroll University and lives in Ohio with her husband Brad and daughters Kate (17), and Sophia (13) who was born with PWS.
Jennifer Bolander has been serving as a Special Education Specialist for PWSA (USA) since October of 2015. She is a graduate of John Carroll University and lives in Ohio with her husband Brad and daughters Kate (17), and Sophia (13) who was born with PWS. Dr. Amy McTighe is the PWS Program Manager and Inpatient Teacher at the Center for Prader-Willi Syndrome at the Children’s Institute of Pittsburgh. She graduated from Duquesne University receiving her Bachelor’s and Master’s degree in Education with a focus on elementary education, special education, and language arts.
Dr. Amy McTighe is the PWS Program Manager and Inpatient Teacher at the Center for Prader-Willi Syndrome at the Children’s Institute of Pittsburgh. She graduated from Duquesne University receiving her Bachelor’s and Master’s degree in Education with a focus on elementary education, special education, and language arts. Evan has worked with the Prader-Willi Syndrome Association (USA) since 2007 primarily as a Crisis Intervention and Family Support Counselor. Evans works with parents and schools to foster strong collaborative relationships and appropriate educational environments for students with PWS.
Evan has worked with the Prader-Willi Syndrome Association (USA) since 2007 primarily as a Crisis Intervention and Family Support Counselor. Evans works with parents and schools to foster strong collaborative relationships and appropriate educational environments for students with PWS. Staci Zimmerman works for Prader-Willi Syndrome Association of Colorado as an Individualized Education Program (IEP) consultant. Staci collaborates with the PWS multi-disciplinary clinic at the Children’s Hospital in Denver supporting families and school districts around the United States with their child’s Individual Educational Plan.
Staci Zimmerman works for Prader-Willi Syndrome Association of Colorado as an Individualized Education Program (IEP) consultant. Staci collaborates with the PWS multi-disciplinary clinic at the Children’s Hospital in Denver supporting families and school districts around the United States with their child’s Individual Educational Plan. Founded in 2001, SDLC is a non-profit legal services organization dedicated to protecting and advancing the legal rights of people with disabilities throughout the South. It partners with the Southern Poverty Law Center, Protection and Advocacy (P&A) programs, Legal Services Corporations (LSC) and disability organizations on major, systemic disability rights issues involving the Individuals with Disabilities Education Act (IDEA), Americans with Disabilities Act (ADA), and the federal Medicaid Act. Recently in November 2014, Jim retired.
Founded in 2001, SDLC is a non-profit legal services organization dedicated to protecting and advancing the legal rights of people with disabilities throughout the South. It partners with the Southern Poverty Law Center, Protection and Advocacy (P&A) programs, Legal Services Corporations (LSC) and disability organizations on major, systemic disability rights issues involving the Individuals with Disabilities Education Act (IDEA), Americans with Disabilities Act (ADA), and the federal Medicaid Act. Recently in November 2014, Jim retired.