As children, we always remember our favorite gifts bestowed at holiday times: a shiny red bike, a beloved doll, a new baseball glove. These gifts and the loved ones who gave them to us are forever etched in our memories now and always. As the holiday season begins and the new year approaches, PWSA | USA is...
Category: News
Looking for a Way to Get Involved? PWSA | USA is in Search of a Volunteer Treasurer
We know that many of you want to make a difference in the lives of our PWS Community – and we have the perfect opportunity if you, or one of your family or friends, are a CPA. Below, you will find the full description of and needs that come with this volunteer position. The treasurer...
PWSA | USA Announced as Harmony Biosciences’ 2023 Patients at the Heart Grant Recipient
About Harmony Biosciences Patients at the Heart Grant Via Harmony Biosciences Press Release: Harmony Biosciences Holdings, Inc. (“Harmony”) (Nasdaq: HRMY), a pharmaceutical company dedicated to developing and commercializing innovative therapies for patients with rare neurological diseases, has selected the latest round of nonprofit organizations for its Patients at the Heart and Progress at the Heart...
PWSA | USA Advocates will Participate in Groundbreaking Opportunity for PWS Community!
U.S. Senate Committee Hearing Live Broadcast Link Now Available This Thursday, PWSA | USA advocates will participate in a groundbreaking opportunity for the PWS community! On October 26, 2023, our advocates will be present for a press conference on Capitol Hill, hosted by Senator Mike Braun (R-IN), Senator Kirsten Gillibrand (D-NY), Representative Mike Gallagher (R-WI),...
Soleno Therapeutics Reports Positive Results from DCCR Study C602 for Prader-Willi Syndrome
Soleno Therapeutics, Inc. has revealed positive outcomes from the randomized withdrawal phase of Study C602, an extended treatment study of DCCR (Diazoxide Choline) Extended-Release tablets for Prader-Willi syndrome (PWS). The results, which showed a significant improvement in hyperphagia-related behaviors in the DCCR group compared to the placebo group, support Soleno's plan to submit a New...
Harmony Biosciences Issues Statement Regarding Confidence in Pitolisant Drug
Harmony Biosciences has reaffirmed its confidence in the strength of WAKIX® (Pitolisant) patents, after receiving a positive ruling from the U.S. Patent and Trademark Office (USPTO) rejecting the request for reexamination. WAKIX® is used to treat excessive daytime sleepiness (EDS) or cataplexy in adults with Narcolepsy. Read Harmony Biosciences' community-facing statement below: We are pleased...
Harmony Biosciences Shares Positive Data from Pitolisant Study at “SLEEP 2023” Annual Meeting
PWSA | USA received information from Harmony Biosciences that the company announced positive findings from the Phase 2 study of its drug pitolisant, a treatment for excessive daytime sleepiness (EDS) in people with Prader-Willi syndrome (PWS). The data was presented at the 37th Annual Meeting of the Associated Professional Sleep Societies (APSS). According to Harmony...
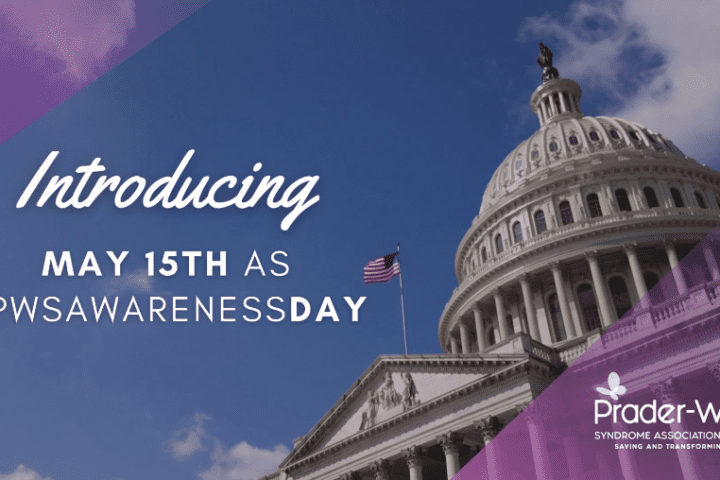
A Historic Milestone for the PWS Community: PWSA | USA Receives Joint Congressional Support to Declare May 15th as National PWS Awareness Day
Today, May 15th, our PWS community has something new to celebrate! PWSA | USA is excited to announce U.S. Congressman Paul D. Tonko (D-NY) and Congresswoman Maria Elvira Salazar (R-FL) have filed a joint resolution declaring May 15, 2023, and every May 15th thereafter, Prader-Willi Syndrome (PWS) Awareness Day in the United States of America!...
Remembering the Life and Impact of Ken Smith
Kenneth (Ken) Smith, 62, of Sarasota, FL, died peacefully on March 7, 2023, at Tidewell Hospice House with his life partner, Patty LaRoche, by his side. He is also survived by his mother and stepfather, Wilma and Clinton Beauford of Pittsburgh, and his sister, Kathy Smith. Ken was preceded in death by his father, Roland...
Harmony Biosciences Shares Statement Regarding Narcolepsy Drug WAKIX
In response to yesterday’s news from Scorpion Capital regarding Harmony Biosciences, we have received the below community-facing statement. Harmony Biosciences shares information about the company’s drug WAKIX, which is used to treat excessive daytime sleepiness (EDS) or cataplexy in adults with Narcolepsy. If you have questions, please reach out to info@pwsausa.org. —————————————————————————- On behalf of...
Harmony Biosciences Encouraged by New Data in Pitolisant Phase 2 Study
This morning, PWSA | USA received new information from Harmony Biosciences regarding its Phase 2 study of pitolisant. This study's purpose is to evaluate the safety and efficacy of the drug in patients with Prader-Willi syndrome (PWS). The company shared the following community-facing statement: "Today, Harmony Biosciences announced topline data on the primary outcome, excessive...
PWSA | USA Announces Keynote Speaker for 2023 National Convention
We are excited to announce the Keynote Speaker for PWSA | USA's 2023 National Convention, Cristol Barrett O’Loughlin who is is the Founder and CEO of A ANGEL AID CARES (Nonprofit Group Enriching Lives, Inc.) You won't want to miss her motivational and inspirational message! Cristol's Keynote speech title is Caring for the Caregivers: From brokenhearted to belonging...
Soleno Therapeutics Announces Start of Randomized Withdrawal Study for PWS Drug DCCR
On Monday, October 3, 2022, PWSA | USA received the news that Soleno Therapeutics will begin its randomized withdrawal study for the drug DCCR, which is being studied as a treatment for Prader-Willi syndrome (PWS). This is a promising next step in Soleno's C602 clinical trial. Soleno shared this randomized withdrawal period will only affect...
Radius Health to Close its RAD011 Clinical Trial
PWSA | USA received the news that Radius Health has decided to end its phase 2/3 clinical trial to evaluate RAD011 as a potential treatment for Prader-Willi syndrome (PWS). Radius was recently purchased by Gurnet Point Capital, who shared it will begin winding down the SCOUT-015 study throughout the month of October. On behalf of...
Radius Health Releases Helpful Resources for SCOUT-015 Trial
In an effort to help our PWS community better understand its SCOUT-015 trial, Radius Health has released several resources about the process of the study, trial sites, and about their drug RAD011 a synthetic cannabidiol oral solution, which is being studied as a possible treatment for hyperphagia and related behaviors in Prader-Willi syndrome. Click on...
Soleno Therapeutics Provides DCCR Update, Continued Communications with FDA
PWSA | USA is happy to share the most recent news from Soleno Therapeutics regarding DCCR: This morning, Soleno shared that through continued dialogue with the FDA, the FDA acknowledged the data from a proposed randomized withdrawal phase of Study C602 would have the potential to address its concerns regarding the adequacy of the overall...
Kasey Bedard, Ph.D., BCBA-D, IBA Shares Findings from Research Study Funded by PWSA | USA
PWSA | USA is excited to share the findings from a grant funding opportunity, awarded to and studied by Kasey Bedard, Ph.D., BCBA-D, IBA. This grant assisted Kasey with her work on PWS Smart-Start, a behavior-analytic caregiver training program. Kasey gives a brief overview of her results below. ------------------------------------------------------------------------------------------ Contributed by Kasey Bedard The purpose...
Saniona Pausing All Clinical Trials for PWS Drug Tesomet Due to Funding Limitations
This morning, Saniona announced they will be voluntarily pausing all Phase 2b Clinical Trials for the drug Tesomet, which is being studied as a treatment for Prader-Willi syndrome (PWS). Saniona has explained that this pause is due to funding limitations and has nothing to do with the "safety or efficacy" of the drug itself. PWSA...
PWSA | USA’s 2021 Annual Report
Dear friends, On behalf of PWSA | USA's Board of Directors and Staff, we are sincerely grateful for YOU, our PWS community, for coming together in advocacy, family support, and research over the past year. We would not be able to offer the resources, care, and hope to our families and individuals living with PWS...
PWSA | USA’s Board of Directors Transition
Each year the Board of Directors says goodbye to the members whose term expires at the end of August, and welcomes all newly-elected or appointed members whose terms begin on September 1. PWSA |USA congratulates and welcomes incoming Board members who began their 2021-2024 term: Clint Hurdle John Lens Marguerite Rupnow (incumbent) Ann Scheimann, M.D....
FDA Advisory Committee to Review LEVO’s Carbetocin as a Treatment for PWS
We are excited to share that Levo’s New Drug Application (NDA) for carbetocin as a treatment for PWS will be discussed at a public meeting of the Psychopharmacologic Drugs Advisory Committee to be held November 4, 2021. This is a major step forward on this drug's path through the approval process. The Food and Drug...
Soleno Therapeutics Announces Positive Data Showing Continued Significant Improvements in Symptoms of PWS following One Year Treatment with DCCR
(Soleno Therapeutics Press Release) Statistically significant reduction in hyperphagia and all other PWS behavioral parameters in Study C602 Statistically significant improvements compared to natural history of PWS from the PATH for PWS Study On track for data submission to the FDA in Q3 2021 REDWOOD CITY, Calif., Sept. 08, 2021 (GLOBE NEWSWIRE) -- Soleno Therapeutics,...
A Message from PWSA | USA’s CEO Paige Rivard Regarding Dr. van Bosse
A message from PWSA | USA’s CEO Paige Rivard: As many of you have heard, Dr. Harold van Bosse is no longer with Shriners Hospitals for Children in Philadelphia. I was in contact with Dr. van Bosse who said he is in the process of moving his practice and is still very committed to the...
Harmony Biosciences Acquires Asset with Novel Mechanism of Action for the Potential Treatment of Narcolepsy and other Rare Neurological Diseases
Harmony Biosciences recently announced the acquisition of HBS-102, a potential first-in-class molecule with a novel mechanism of action, from ConSynance Therapeutics, Inc. HBS-102 is a Melanin Concentrating Hormone Receptor 1 (MCHR1) antagonist that has the potential to offer a novel approach to the treatment of narcolepsy including the symptoms of Rapid Eye Movement (REM) sleep...




















 Jennifer Bolander has been serving as a Special Education Specialist for PWSA (USA) since October of 2015. She is a graduate of John Carroll University and lives in Ohio with her husband Brad and daughters Kate (17), and Sophia (13) who was born with PWS.
Jennifer Bolander has been serving as a Special Education Specialist for PWSA (USA) since October of 2015. She is a graduate of John Carroll University and lives in Ohio with her husband Brad and daughters Kate (17), and Sophia (13) who was born with PWS. Perry A. Zirkel has written more than 1,500 publications on various aspects of school law, with an emphasis on legal issues in special education. He writes a regular column for NAESP’s Principal magazine and NASP’s Communiqué newsletter, and he did so previously for Phi Delta Kappan and Teaching Exceptional Children.
Perry A. Zirkel has written more than 1,500 publications on various aspects of school law, with an emphasis on legal issues in special education. He writes a regular column for NAESP’s Principal magazine and NASP’s Communiqué newsletter, and he did so previously for Phi Delta Kappan and Teaching Exceptional Children. Evan has worked with the Prader-Willi Syndrome Association (USA) since 2007 primarily as a Crisis Intervention and Family Support Counselor. Evans works with parents and schools to foster strong collaborative relationships and appropriate educational environments for students with PWS.
Evan has worked with the Prader-Willi Syndrome Association (USA) since 2007 primarily as a Crisis Intervention and Family Support Counselor. Evans works with parents and schools to foster strong collaborative relationships and appropriate educational environments for students with PWS. Dr. Amy McTighe is the PWS Program Manager and Inpatient Teacher at the Center for Prader-Willi Syndrome at the Children’s Institute of Pittsburgh. She graduated from Duquesne University receiving her Bachelor’s and Master’s degree in Education with a focus on elementary education, special education, and language arts.
Dr. Amy McTighe is the PWS Program Manager and Inpatient Teacher at the Center for Prader-Willi Syndrome at the Children’s Institute of Pittsburgh. She graduated from Duquesne University receiving her Bachelor’s and Master’s degree in Education with a focus on elementary education, special education, and language arts. Staci Zimmerman works for Prader-Willi Syndrome Association of Colorado as an Individualized Education Program (IEP) consultant. Staci collaborates with the PWS multi-disciplinary clinic at the Children’s Hospital in Denver supporting families and school districts around the United States with their child’s Individual Educational Plan.
Staci Zimmerman works for Prader-Willi Syndrome Association of Colorado as an Individualized Education Program (IEP) consultant. Staci collaborates with the PWS multi-disciplinary clinic at the Children’s Hospital in Denver supporting families and school districts around the United States with their child’s Individual Educational Plan. Founded in 2001, SDLC is a non-profit legal services organization dedicated to protecting and advancing the legal rights of people with disabilities throughout the South. It partners with the Southern Poverty Law Center, Protection and Advocacy (P&A) programs, Legal Services Corporations (LSC) and disability organizations on major, systemic disability rights issues involving the Individuals with Disabilities Education Act (IDEA), Americans with Disabilities Act (ADA), and the federal Medicaid Act. Recently in November 2014, Jim retired.
Founded in 2001, SDLC is a non-profit legal services organization dedicated to protecting and advancing the legal rights of people with disabilities throughout the South. It partners with the Southern Poverty Law Center, Protection and Advocacy (P&A) programs, Legal Services Corporations (LSC) and disability organizations on major, systemic disability rights issues involving the Individuals with Disabilities Education Act (IDEA), Americans with Disabilities Act (ADA), and the federal Medicaid Act. Recently in November 2014, Jim retired.