Date: Wednesday, April 16, 2025Time: 9:15 AM PT | 12:15 PM ETLocation: Virtual via ZOOM The Prader-Willi syndrome (PWS) community has reached a historic milestone – VYKAT XR (formerly known as DCCR in clinical trials) is now FDA-approved as the first-ever treatment for hyperphagia in PWS. To help families, caregivers, and healthcare providers understand what...
Category: Medical
Soleno Therapeutics Submits New Drug Application to FDA for PWS Treatment
On June 28, 2024, Soleno Therapeutics announced the company officially submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for DCCR (diazoxide choline) extended-release tablets. This new treatment targets Prader-Willi syndrome (PWS) in individuals aged 4 and older with hyperphagia. CEO Anish Bhatnagar, M.D., says, “Submission of the DCCR NDA to...
Fueling Hope: Aardvark Therapeutics’ $85M Boost Powers Breakthrough PWS Treatment
Aardvark Therapeutics has just hit a major milestone with an exciting $85 million Series C financing round! Led by Decheng Capital, with additional participation from several investors, including PWSA | USA, this funding is set to drive the development of ARD-101, a groundbreaking treatment for hyperphagia in Prader-Willi syndrome patients. ARD-101 showed promising early trial...
Preserving Progress: Stand Up for Fully Funding the BRAIN Initiative!
The NIH-BRAIN Initiative (Brain Research Through Advancing Innovative Neurotechnologies – Initiative), is a partnership between the National Institutes of Health (NIH) and nonfederal funding sources to advance innovative neurotechnologies to revolutionize the understanding of the brain/neurocircuitry. Since its inception in 2013, this program has had important impacts on our understanding of normal brain function this...
Pitolisant Shows Positive Secondary Outcomes in Phase 2 Study
Exciting news for the Prader-Willi syndrome (PWS) community! Newly unveiled data from Harmony Biosciences' phase 2 study reveals promising impacts of pitolisant (Wakix) on PWS patients with excessive daytime sleepiness (EDS). Harmony Biosciences plans to kick off its phase 3 TEMPO study later this year, aiming to dive deeper into the potential of this treatment...
Acadia Pharmaceuticals Unveils New COMPASS PWS Study Website
This new website gives in-depth details about Acadia Pharmaceuticals COMPASS PWS Study, including a brief video about the study, eligibility for participation in the study, what to expect at study visits, and a research site locator. Click the button below to enter the website. ABOUT THE COMPASS PWS STUDY: The COMPASS PWS study aims to...
Harmony Biosciences Reveals Encouraging Data from Phase 2 Pitolisant Study in PWS Patients
Harmony Biosciences has presented new secondary endpoint data concerning the use of pitolisant, an investigational drug, for the treatment of excessive daytime sleepiness (EDS) in Prader-Willi syndrome. According to a recent press release Harmony Biosciences released, the Phase 2 signal-detection study showed improvements in behavioral disturbances, especially in the higher-dose pitolisant group, as well as...
Soleno Therapeutics Reports Positive Results from DCCR Study C602 for Prader-Willi Syndrome
Soleno Therapeutics, Inc. has revealed positive outcomes from the randomized withdrawal phase of Study C602, an extended treatment study of DCCR (Diazoxide Choline) Extended-Release tablets for Prader-Willi syndrome (PWS). The results, which showed a significant improvement in hyperphagia-related behaviors in the DCCR group compared to the placebo group, support Soleno's plan to submit a New...
Neuren Pharmaceuticals is Happy to Announce the First Site Participating in Their Phase II, Open Label, PWS Study (Neu-2591-PWS-001) is Now Open for Screening!
Important information regarding this exciting milestone: Rare Disease Research (RDR), located in Atlanta, GA, is now welcoming children with PWS and their families to their clinicfor screening into this trial. The duration of active treatment in this study is 13 weeks. In a preclinical study in animals, physiological and behavioral symptoms were normalized within six...
Gedeon Richter Now Recruiting for KITE-PWS Clinical Trial
You or someone you love could be part of developing new therapies for Prader-Willi syndrome. Learn about Gedeon Richter's research study KITE-PWS, also known as RGH-706-003, to evaluate an experimental drug for hyperphagia in people with Prader-Willi syndrome. The oral drug RGH-706 works by blocking melanin-concentrating hormone (MCH), which is a key part in the...
Harmony Biosciences Encouraged by New Data in Pitolisant Phase 2 Study
This morning, PWSA | USA received new information from Harmony Biosciences regarding its Phase 2 study of pitolisant. This study's purpose is to evaluate the safety and efficacy of the drug in patients with Prader-Willi syndrome (PWS). The company shared the following community-facing statement: "Today, Harmony Biosciences announced topline data on the primary outcome, excessive...
It’s Flu and RSV Season – How to Protect Your Child
Contributed by PWSA | USA's Family Support Team The Centers for Disease Control and Prevention (CDC) has shown an increase in Respiratory Syncytial Virus (RSV) infections in multiple areas of the US https://www.cdc.gov/rsv/index.html. RSV infection can cause a variety of respiratory illnesses in infants and young children. It most commonly causes a cold-like illness but...
Radius Health Releases Helpful Resources for SCOUT-015 Trial
In an effort to help our PWS community better understand its SCOUT-015 trial, Radius Health has released several resources about the process of the study, trial sites, and about their drug RAD011 a synthetic cannabidiol oral solution, which is being studied as a possible treatment for hyperphagia and related behaviors in Prader-Willi syndrome. Click on...
Soleno Therapeutics Provides DCCR Update, Continued Communications with FDA
PWSA | USA is happy to share the most recent news from Soleno Therapeutics regarding DCCR: This morning, Soleno shared that through continued dialogue with the FDA, the FDA acknowledged the data from a proposed randomized withdrawal phase of Study C602 would have the potential to address its concerns regarding the adequacy of the overall...
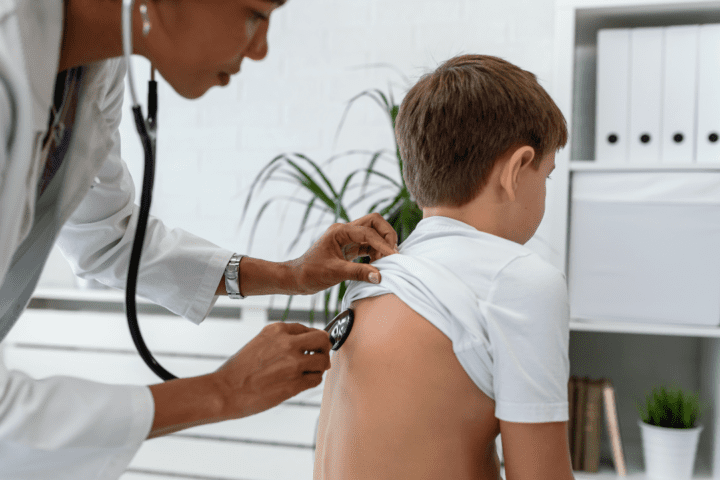
Hyperphagia and How it Affects Learning
Contributed by Stacy Ward, MS Director of Family Support and Lynn Garrick, RN, BSN Medical/Research Coordinator Prader-Willi syndrome (PWS) is a rare neurodevelopmental genetic disorder that affects multiple systems in the body. There are many symptoms of PWS, including hyperphagia, behavioral challenges, hypotonia, incomplete sexual development, cognitive deficits, metabolic dysregulation, and several more. Hyperphagia is...
Clinical Trial Sites Announced for Radius Health’s RAD011 SCOUT-015 Study
Radius Health has announced the locations for its RAD011 SCOUT-015 Clinical Trials. Below, you will find a downloadable map with each participating hospital and medical center around the country. The RAD011 SCOUT-015 studies are in various stages, depending on the location, from initiated to activated and ready to enroll. You can also find a comprehensive...
World Osteoporosis Day
Read PWSA | USA Clinical Advisory Board's full Consensus Statement Here. Wednesday, October 20th is recognized as World Osteoporosis Day. Osteoporosis is a condition that commonly impacts individuals living with Prader-Willi syndrome, and is typically diagnosed in adolescence and adulthood. The cause(s) of the osteoporosis is not totally clear, but it is thought to be...
Summary of a Streamlined Molecular Diagnostic Approach for Prader-Willi and Other Related Syndromes
Written by: Merlin G. Butler, MD, PhD Historically, to confirm the diagnosis and molecular genetic classes in Prader Willi syndrome (PWS) required a stepwise approach using multiple methods needing more time and resources. Due to advances in genetic testing and availability of multiple analytical methodologies, a streamlined approach was developed and reported by Strom et...
Soleno Therapeutics Announces Positive Data Showing Continued Significant Improvements in Symptoms of PWS following One Year Treatment with DCCR
(Soleno Therapeutics Press Release) Statistically significant reduction in hyperphagia and all other PWS behavioral parameters in Study C602 Statistically significant improvements compared to natural history of PWS from the PATH for PWS Study On track for data submission to the FDA in Q3 2021 REDWOOD CITY, Calif., Sept. 08, 2021 (GLOBE NEWSWIRE) -- Soleno Therapeutics,...
Radius Health Announces Plans for Global Prader-Willi Syndrome Pivotal Study
On behalf of Radius Health, Inc: Boston, Mass., July 22, 2021 — Radius Health, Inc. announced Wednesday, July 21, 2021 that it has recently received the written meeting minutes from a June Type C meeting held with the U.S. Food and Drug Administration (FDA) regarding RAD011, a synthetic cannabidiol oral solution. RAD011 is initially to...
Soleno Therapeutics Provides Update on DCCR for the Treatment of PWS
FDA agrees to review additional data to determine adequacy for submission of NDA Soleno Therapeutics, Inc. provided an update on Tuesday, July 7, 2021 following a recent interaction with the FDA regarding the development of DCCR, a potential treatment for PWS. According to Soleno Therapeutics, on July 2, 2021 they received news from The FDA,...
FDA Grants Priority Review for Levo Therapeutics’ New Drug Application for LV-101 (Intranasal Carbetocin) for the Treatment of Prader-Willi Syndrome
Read Levo Therapeutic, Inc.’s Full Article HERE. CHICAGO, IL, July 6, 2021 (Newswire.com) – Levo Therapeutics, Inc., a biotechnology company dedicated to using genetic insights to advance treatments for Prader-Willi syndrome (PWS) and related disorders, announced today that the U.S. Food and Drug Administration (FDA) has accepted its New Drug Application (NDA) for review and...
Sleep Disturbances in Prader-Willi Syndrome
Sleep health is essential for everyone; it is just as important to take care of ourselves as parents and caregivers as it is for those living with Prader-Willi syndrome (PWS). We understand that disordered sleep has implications for cognitive outcomes, mental and physical health, and work and school performance. Sleep disturbances can occur from many...
News Release: Rhythm Wins FDA Approval for Obesity Drug Imcivree
Rhythm Pharmaceuticals Inc., a company developing medicines for rare genetic disorders of obesity, has won FDA approval for its first product, Imcivree (setmelanotide), following a priority review. The drug is designed to restore a biological pathway that, when disrupted, can lead to constant hunger. The approval covers the treatment of three types of ultrarare early...




















 Jennifer Bolander has been serving as a Special Education Specialist for PWSA (USA) since October of 2015. She is a graduate of John Carroll University and lives in Ohio with her husband Brad and daughters Kate (17), and Sophia (13) who was born with PWS.
Jennifer Bolander has been serving as a Special Education Specialist for PWSA (USA) since October of 2015. She is a graduate of John Carroll University and lives in Ohio with her husband Brad and daughters Kate (17), and Sophia (13) who was born with PWS. Perry A. Zirkel has written more than 1,500 publications on various aspects of school law, with an emphasis on legal issues in special education. He writes a regular column for NAESP’s Principal magazine and NASP’s Communiqué newsletter, and he did so previously for Phi Delta Kappan and Teaching Exceptional Children.
Perry A. Zirkel has written more than 1,500 publications on various aspects of school law, with an emphasis on legal issues in special education. He writes a regular column for NAESP’s Principal magazine and NASP’s Communiqué newsletter, and he did so previously for Phi Delta Kappan and Teaching Exceptional Children. Evan has worked with the Prader-Willi Syndrome Association (USA) since 2007 primarily as a Crisis Intervention and Family Support Counselor. Evans works with parents and schools to foster strong collaborative relationships and appropriate educational environments for students with PWS.
Evan has worked with the Prader-Willi Syndrome Association (USA) since 2007 primarily as a Crisis Intervention and Family Support Counselor. Evans works with parents and schools to foster strong collaborative relationships and appropriate educational environments for students with PWS. Dr. Amy McTighe is the PWS Program Manager and Inpatient Teacher at the Center for Prader-Willi Syndrome at the Children’s Institute of Pittsburgh. She graduated from Duquesne University receiving her Bachelor’s and Master’s degree in Education with a focus on elementary education, special education, and language arts.
Dr. Amy McTighe is the PWS Program Manager and Inpatient Teacher at the Center for Prader-Willi Syndrome at the Children’s Institute of Pittsburgh. She graduated from Duquesne University receiving her Bachelor’s and Master’s degree in Education with a focus on elementary education, special education, and language arts. Staci Zimmerman works for Prader-Willi Syndrome Association of Colorado as an Individualized Education Program (IEP) consultant. Staci collaborates with the PWS multi-disciplinary clinic at the Children’s Hospital in Denver supporting families and school districts around the United States with their child’s Individual Educational Plan.
Staci Zimmerman works for Prader-Willi Syndrome Association of Colorado as an Individualized Education Program (IEP) consultant. Staci collaborates with the PWS multi-disciplinary clinic at the Children’s Hospital in Denver supporting families and school districts around the United States with their child’s Individual Educational Plan. Founded in 2001, SDLC is a non-profit legal services organization dedicated to protecting and advancing the legal rights of people with disabilities throughout the South. It partners with the Southern Poverty Law Center, Protection and Advocacy (P&A) programs, Legal Services Corporations (LSC) and disability organizations on major, systemic disability rights issues involving the Individuals with Disabilities Education Act (IDEA), Americans with Disabilities Act (ADA), and the federal Medicaid Act. Recently in November 2014, Jim retired.
Founded in 2001, SDLC is a non-profit legal services organization dedicated to protecting and advancing the legal rights of people with disabilities throughout the South. It partners with the Southern Poverty Law Center, Protection and Advocacy (P&A) programs, Legal Services Corporations (LSC) and disability organizations on major, systemic disability rights issues involving the Individuals with Disabilities Education Act (IDEA), Americans with Disabilities Act (ADA), and the federal Medicaid Act. Recently in November 2014, Jim retired.