IPWSO 2022 - Call for Abstracts: Professional Providers and Caregivers - 7 & 8 July 2022, University of Limerick, Ireland. Please spread the word! The IPWSO 2022 Professional Providers and Caregivers Conference is now calling for abstracts for presentations to be submitted for the Conference on 7 and 8 July 2022 at the University of...
Donor Spotlight: Elaine Towle
PWSA | USA is special because of YOU, our donors, who passionately give to support our mission. It is because of your generous gifts that we can provide help and hope through family support, advocacy, and research efforts. To celebrate and honor your generosity, each month we will spotlight a donor and their story showcasing...
PWSA | USA Board Member Clint Hurdle Accepts Position with Colorado Rockies as Assistant GM
December 13, 2021 -- We are very proud and excited for PWSA | USA Board of Directors member Clint Hurdle as he makes a return to the Colorado Rockies baseball team and takes on the role of Assistant to the General Manager. Clint is a longtime supporter of PWSA | USA and has been an...
The Chicago School of Professional Psychology is looking for Research Study Participants
YOU can help the Chicago School of Professional Psychology learn about the effects of a behavioral caregiver training program for caregivers of children with PWS. Participants will be compensated with a $500 Visa gift card following the completion of the study. Parents with children with multiple diagnoses can reach out to Dr. Bedard for clarification...
Research Survey Opportunity: Behavioral Supports and Services for Children with Prader-Willi Syndrome
Are you a caregiver of at least one child, of any age, who has been diagnosed with Prader-Willi Syndrome? Do you have access to the internet and an internet-connected device? Can you read and write in English? Are you over the age of 18? If you answered YES to these questions, you are invited to...
Giving Tuesday: Here’s How You Can Support PWSA | USA
Are you planning on making a year-end gift? Giving Tuesday is just one week away and is the perfect opportunity to show your support! Thanks to the generosity of an anonymous donor, your Giving Tuesday donation will be MATCHED dollar for dollar up to $13,000. Make a gift on November 30, 2021 and give twice the good...
Bid on Dozens of Incredible Angel Drive Auction Items
We are excited to share that the 2021 PWSA | USA Angel Drive Auction is now LIVE, and just in time for the holidays! Browse through our incredible items and place your bids TODAY! Funds from the auction support our 2021 Angel Drive. Together, we can help to make sure all families have the support...
8th Annual Clint Hurdle Hot Stove Dinner 2022: Save the Date!
We are excited to announce the date for the 8th Annual Clint Hurdle Hot Stove Dinner. Please mark your calendars for March 26, 2022 and save the date to join us on Anna Maria Island or online! Sip, Savor, and Support Prader-Willi Syndrome Association | USA while enjoying the outdoor event on the beautiful grounds...
My Sister Linda
Linda was the oldest, born in 1951 in New York City. Prader-Willi syndrome wasn’t discovered until 1956. My parents noticed Linda would go to the refrigerator often and that she was a bit short for her age. We moved to San Diego in 1961. Linda loved playing tennis, swimming, and eating out. She especially loved...
Donor Spotlight: Michelle Spring
PWSA | USA is special because of YOU, our donors, who passionately give to support our mission. It is because of your generous gifts that we can provide help and hope through family support, advocacy, and research efforts. To celebrate and honor your generosity, each month we will spotlight a donor and their story showcasing...
In the Brain’s Cerebellum, a New Target for Suppressing Hunger
Peer-Reviewed Publication | UNIVERSITY OF PENNSYLVANIA A research team led by the University of Pennsylvania's J. Nicholas Betley has identified an entirely new way the brain signals fullness after eating. The findings offer a novel target for therapies that could dramatically curb overeating. People with Prader-Willi syndrome, a genetic disorder, have an insatiable appetite. They...
National Adoption Day: Luke’s Story
Hello, we are Gabriel and Sarah Hahn. We knew before we married that we desired adoption to be a part of how we built our family. After the birth of our fourth biological child, we entered the world of adoption. Over the last seven years, we have been humbled to have been chosen on three...
National Adoption Day: Michael’s Story
After almost ten years of attempting to become parents via fertility treatments, failed foster care adoption committees and even an adoption facilitator scam, we learned about a very special little two-month-old baby boy named Baby M, who had been diagnosed with Prader Willi Syndrome, through Special Angels Adoption Agency. He had been born at Yale...
Spotlight on Hope: Isaac Davis
We are starting a new initiative to inspire HOPE for our PWS community! Spotlight on Hope will share stories about individuals living with PWS: stories about a recent triumph, big or small, overcoming a challenge, being recognized in their community, or anything that inspires hope or joy in their life and those around them. If...
Community Conversation: School Success Toolkits
REGISTER IN ADVANCE HERE
Join PWSA | USA’s Advocacy Committee!
The PWSA | USA Advocacy Committee is opening spots for NEW MEMBERS to join. The purpose of the PWSA | USA Advocacy Committee is to advise and educate the PWS Community on public policy issues related to PWS and the rare disease community. In addition, the committee is responsible for providing policy direction in the...
Join PWSA | USA, FPWR in Combined Community Conversation to Discuss FDA Advisory Committee’s Decision on LV-101 Carbetocin Nasal Spray
PWSA | USA and FPWR will host a combined Community Conversation on Tuesday, November 9th at 8:00 p.m. EST. PWSA | USA CEO Paige Rivard and FPWR Director of Research Programs Theresa Strong will discuss the outcome of the November 4th FDA Advisory Committee Meeting regarding LV-101 carbetocin nasal spray. This will serve as an...
FDA Advisory Committee Meeting Outcome: A Message from Paige Rivard, Levo CEO Sara Cotter
"On Thursday, November 4, 2021, an FDA Advisory Committee meeting was held for LV-101 carbetocin nasal spray. The Advisory Committee placed their vote, and unfortunately, it was 12 (No) and one (Yes) based on the one question - Has the applicant provided substantial evidence of effectiveness for carbetocin nasal spray (LV-101) in the treatment of...
Donor Spotlight: John Lens
PWSA | USA is special because of YOU, our donors, who passionately give to support our mission. It is because of your generous gifts that we can provide help and hope through family support, advocacy, and research efforts. To celebrate and honor your generosity, each month we will spotlight a donor and their story showcasing...
How to Watch the FDA Advisory Committee Meeting
On Thursday, November 4, 2021 from 8:45 a.m. to 3:30 p.m. EST, the U.S. Food and Drug Administration (FDA) will hold an Advisory Committee Meeting to discuss the new drug application (NDA) 214812, for carbetocin nasal spray, submitted by Levo Therapeutics, Inc., for the proposed treatment of hyperphagia, anxiety, and distress behaviors associated with Prader-Willi...
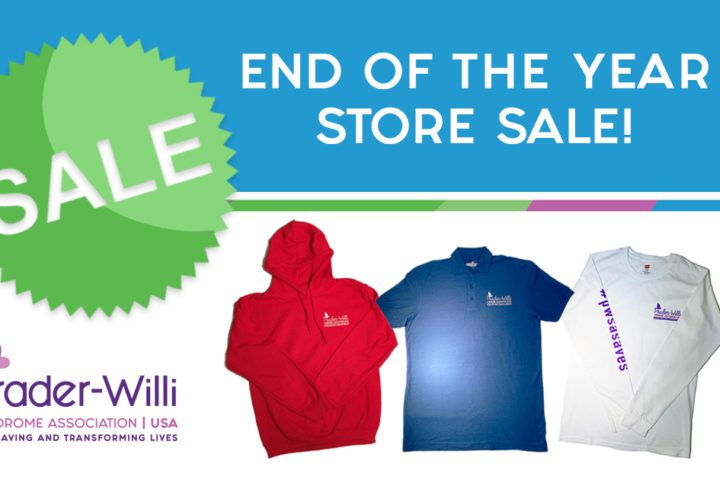
PWSA | USA End of the Year Clothing Sale is LIVE
Start your holiday shopping with PWSA | USA! Through December 31st, PWSA | USA clothing items in our online store are available for purchase at 50% OFF and our Eddie Bauer Trail Jacket is on sale for 40% off. Learn more and visit the shop by clicking the button below!
Prolapsed Rectum and Risk Factors in Prader–Willi Syndrome: A Case-Based Review
Written by: Merlin G. Butler ABSTRACT A 14-year-old boy with Prader–Willi syndrome (PWS) with maternal disomy 15 is reported with rectal prolapse as only the second patient in the literature. With predisposing risk factors present for rectal damage and prolapse in this syndrome, the incidence must be higher and therefore underreported. These risk factors include...
Show Your Support for the 2021 Angel Drive!
Due to the generosity of our donors, your donation to the Angel Drive will now be matched dollar for dollar up to $60,000. Help us reach our goal of raising $120,000 by December 31, 2021! The 2021 PWSA | USA Angel Drive kicks off TODAY and we are counting on YOU to be our partner...
PWSA | USA’s 2021 Angel Drive Campaigns Kicks Off November 1st!
The 2021 Angel Drive Campaign kicks off November 1st and we're counting on YOU to be our partner in hope! Every year, more than 2,000 individuals and families turn to PWSA | USA to find help when they need it most. Ours is the only organization that provides the comprehensive support, tools, and resources families need...





















 Jennifer Bolander has been serving as a Special Education Specialist for PWSA (USA) since October of 2015. She is a graduate of John Carroll University and lives in Ohio with her husband Brad and daughters Kate (17), and Sophia (13) who was born with PWS.
Jennifer Bolander has been serving as a Special Education Specialist for PWSA (USA) since October of 2015. She is a graduate of John Carroll University and lives in Ohio with her husband Brad and daughters Kate (17), and Sophia (13) who was born with PWS. Perry A. Zirkel has written more than 1,500 publications on various aspects of school law, with an emphasis on legal issues in special education. He writes a regular column for NAESP’s Principal magazine and NASP’s Communiqué newsletter, and he did so previously for Phi Delta Kappan and Teaching Exceptional Children.
Perry A. Zirkel has written more than 1,500 publications on various aspects of school law, with an emphasis on legal issues in special education. He writes a regular column for NAESP’s Principal magazine and NASP’s Communiqué newsletter, and he did so previously for Phi Delta Kappan and Teaching Exceptional Children. Evan has worked with the Prader-Willi Syndrome Association (USA) since 2007 primarily as a Crisis Intervention and Family Support Counselor. Evans works with parents and schools to foster strong collaborative relationships and appropriate educational environments for students with PWS.
Evan has worked with the Prader-Willi Syndrome Association (USA) since 2007 primarily as a Crisis Intervention and Family Support Counselor. Evans works with parents and schools to foster strong collaborative relationships and appropriate educational environments for students with PWS. Dr. Amy McTighe is the PWS Program Manager and Inpatient Teacher at the Center for Prader-Willi Syndrome at the Children’s Institute of Pittsburgh. She graduated from Duquesne University receiving her Bachelor’s and Master’s degree in Education with a focus on elementary education, special education, and language arts.
Dr. Amy McTighe is the PWS Program Manager and Inpatient Teacher at the Center for Prader-Willi Syndrome at the Children’s Institute of Pittsburgh. She graduated from Duquesne University receiving her Bachelor’s and Master’s degree in Education with a focus on elementary education, special education, and language arts. Staci Zimmerman works for Prader-Willi Syndrome Association of Colorado as an Individualized Education Program (IEP) consultant. Staci collaborates with the PWS multi-disciplinary clinic at the Children’s Hospital in Denver supporting families and school districts around the United States with their child’s Individual Educational Plan.
Staci Zimmerman works for Prader-Willi Syndrome Association of Colorado as an Individualized Education Program (IEP) consultant. Staci collaborates with the PWS multi-disciplinary clinic at the Children’s Hospital in Denver supporting families and school districts around the United States with their child’s Individual Educational Plan. Founded in 2001, SDLC is a non-profit legal services organization dedicated to protecting and advancing the legal rights of people with disabilities throughout the South. It partners with the Southern Poverty Law Center, Protection and Advocacy (P&A) programs, Legal Services Corporations (LSC) and disability organizations on major, systemic disability rights issues involving the Individuals with Disabilities Education Act (IDEA), Americans with Disabilities Act (ADA), and the federal Medicaid Act. Recently in November 2014, Jim retired.
Founded in 2001, SDLC is a non-profit legal services organization dedicated to protecting and advancing the legal rights of people with disabilities throughout the South. It partners with the Southern Poverty Law Center, Protection and Advocacy (P&A) programs, Legal Services Corporations (LSC) and disability organizations on major, systemic disability rights issues involving the Individuals with Disabilities Education Act (IDEA), Americans with Disabilities Act (ADA), and the federal Medicaid Act. Recently in November 2014, Jim retired.