Neuren Pharmaceuticals Opens Second Trial Site for Phase II PWS Study
Neuren Pharmaceuticals is pleased to announce their second site participating in their Phase II, Open Label, PWS Study (Neu-2591-PWS-001) is now open for screening! Important information regarding this exciting milestone: Two sites are now open to enrollment! Rare Disease Research (RDR), located in Atlanta, GA, and Uncommon Cures, located in Chevy Chase, MD (8 miles outside...
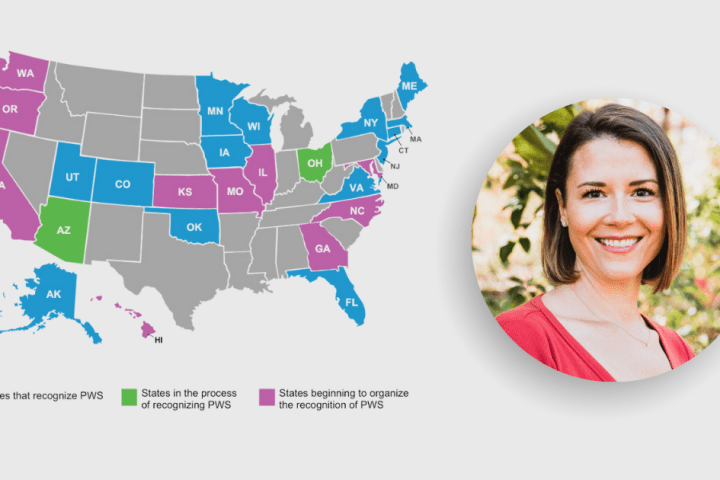
Joint Legislation Filed to have PWS Recognized as a Developmental Disability in Ohio
PWSA | USA is excited to announce that thanks to the dedication and hard work of our Advocacy Ambassador, Dr. Erin Carter-Cooper, PhD, Ohio Representative Rose Sweeney (D - District 16) and Representative Gayle Mannin (R – District 52) have jointly filed legislation that will make Prader-Willi syndrome a recognized developmental disability in the State of Ohio!...
2023 Operation Holiday Cheer Applications Now Being Accepted
Due to the generosity of an anonymous donor’s gift, PWSA | USA is once again able to bring holiday cheer to families in need this holiday season! We will identify a select number of families to receive gift cards to be used to help ease the financial stresses of the holidays. To be considered, please...
Give the Gift of Hope During PWSA | USA’s 2023 Angel Drive Campaign!
As children, we always remember our favorite gifts bestowed at holiday times: a shiny red bike, a beloved doll, a new baseball glove. These gifts and the loved ones who gave them to us are forever etched in our memories now and always. As the holiday season begins and the new year approaches, PWSA | USA is...




 Perry A. Zirkel has written more than 1,500 publications on various aspects of school law, with an emphasis on legal issues in special education. He writes a regular column for NAESP’s Principal magazine and NASP’s Communiqué newsletter, and he did so previously for Phi Delta Kappan and Teaching Exceptional Children.
Perry A. Zirkel has written more than 1,500 publications on various aspects of school law, with an emphasis on legal issues in special education. He writes a regular column for NAESP’s Principal magazine and NASP’s Communiqué newsletter, and he did so previously for Phi Delta Kappan and Teaching Exceptional Children. Jennifer Bolander has been serving as a Special Education Specialist for PWSA (USA) since October of 2015. She is a graduate of John Carroll University and lives in Ohio with her husband Brad and daughters Kate (17), and Sophia (13) who was born with PWS.
Jennifer Bolander has been serving as a Special Education Specialist for PWSA (USA) since October of 2015. She is a graduate of John Carroll University and lives in Ohio with her husband Brad and daughters Kate (17), and Sophia (13) who was born with PWS. Dr. Amy McTighe is the PWS Program Manager and Inpatient Teacher at the Center for Prader-Willi Syndrome at the Children’s Institute of Pittsburgh. She graduated from Duquesne University receiving her Bachelor’s and Master’s degree in Education with a focus on elementary education, special education, and language arts.
Dr. Amy McTighe is the PWS Program Manager and Inpatient Teacher at the Center for Prader-Willi Syndrome at the Children’s Institute of Pittsburgh. She graduated from Duquesne University receiving her Bachelor’s and Master’s degree in Education with a focus on elementary education, special education, and language arts. Evan has worked with the Prader-Willi Syndrome Association (USA) since 2007 primarily as a Crisis Intervention and Family Support Counselor. Evans works with parents and schools to foster strong collaborative relationships and appropriate educational environments for students with PWS.
Evan has worked with the Prader-Willi Syndrome Association (USA) since 2007 primarily as a Crisis Intervention and Family Support Counselor. Evans works with parents and schools to foster strong collaborative relationships and appropriate educational environments for students with PWS. Staci Zimmerman works for Prader-Willi Syndrome Association of Colorado as an Individualized Education Program (IEP) consultant. Staci collaborates with the PWS multi-disciplinary clinic at the Children’s Hospital in Denver supporting families and school districts around the United States with their child’s Individual Educational Plan.
Staci Zimmerman works for Prader-Willi Syndrome Association of Colorado as an Individualized Education Program (IEP) consultant. Staci collaborates with the PWS multi-disciplinary clinic at the Children’s Hospital in Denver supporting families and school districts around the United States with their child’s Individual Educational Plan. Founded in 2001, SDLC is a non-profit legal services organization dedicated to protecting and advancing the legal rights of people with disabilities throughout the South. It partners with the Southern Poverty Law Center, Protection and Advocacy (P&A) programs, Legal Services Corporations (LSC) and disability organizations on major, systemic disability rights issues involving the Individuals with Disabilities Education Act (IDEA), Americans with Disabilities Act (ADA), and the federal Medicaid Act. Recently in November 2014, Jim retired.
Founded in 2001, SDLC is a non-profit legal services organization dedicated to protecting and advancing the legal rights of people with disabilities throughout the South. It partners with the Southern Poverty Law Center, Protection and Advocacy (P&A) programs, Legal Services Corporations (LSC) and disability organizations on major, systemic disability rights issues involving the Individuals with Disabilities Education Act (IDEA), Americans with Disabilities Act (ADA), and the federal Medicaid Act. Recently in November 2014, Jim retired.